how to find ionization energy
This difference is the ionization energy for that ion. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells.

Ionization Energy And Electronegativity

Ionization Energies From Ground State Energy Differences For The

04 Periodic Trends V2
Ionization energy and atomic number.
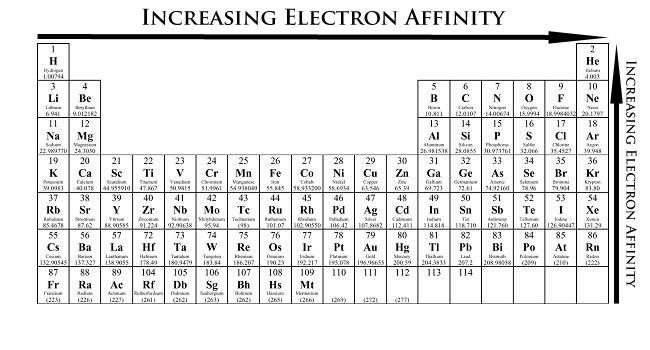
How to find ionization energy on periodic table.
This video explains the major periodic table trends such as.
How to calculate the ionization energy of atoms determine what atom you want to use for calculating the ionization energy.
You must first find the energy value of the ion you are looking for.
The process by which the first ionization energy of hydrogen is measured would be represented by the following equation.
We can explain this by considering the nuclear charge of the atom.
This list contains the 118 elements of chemistry.
For atoms with more than.
For example sodium requires only 496 kjmol or 514 evatom to ionize it.
I also go over the exceptions.
The more protons in the nucleus the stronger the attraction of the nucleus to electrons.
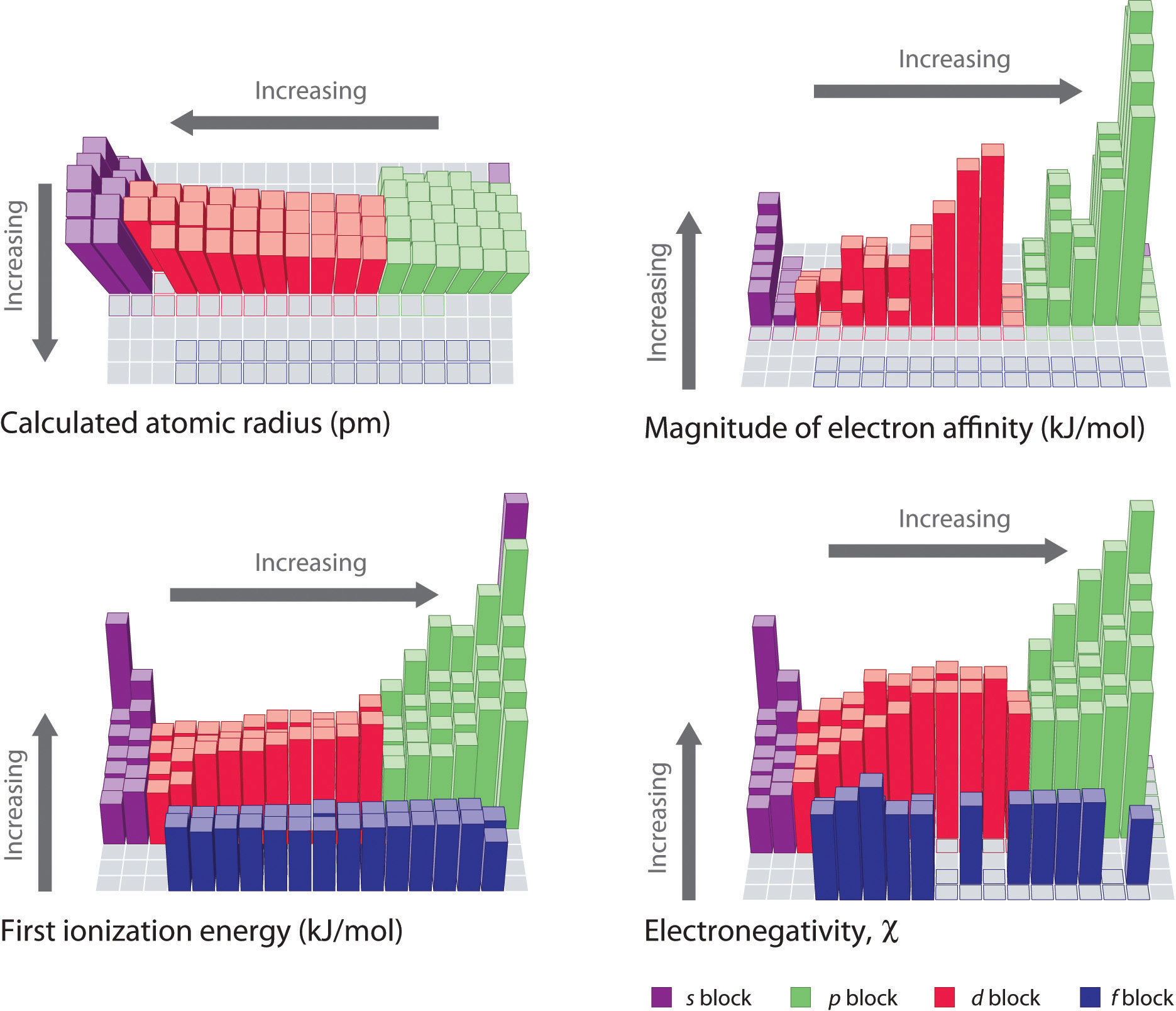
Moving from left to right across the periodic table the ionization energy for an atom increases.
The first ionization energy is the energy required to remove one electron from the parent atom.
When looking at a periodic table ionization energy generally decreases from the top to the bottom of the chart and increases from the left to the right of the chart.
Electronegativity ionization energy electron affinity atomic radius ion size and metallic character.
The elements of the periodic table sorted by ionization energy.
On the other hand neon the noble gas immediately preceding it in the periodic table requires 2081 kjmol or 2156 evatom.
Click on any elements name for further information on chemical properties environmental data or health effects.
Successive ionization energies increase.
The second ionization energy is the energy required to remove a second valence electron from the univalent ion to form the divalent ion and so on.
Decide how many electrons the atom contains.
The first of two main methods which scientists use to calculate the ionization energy is the subtraction method.
The amount of energy necessary to lose one electron from a mole of gas phase atoms is called an elements ionization energy.
Then subtract the energy value of the neutral atom.
By definition the first ionization energy of an element is the energy needed to remove the outermost or highest energy electron from a neutral atom in the gas phase.
This method entails some experimentation.
Calculate the ionization energy in units of electron volts for a one electron atom by squaring z.

The Parts Of The Periodic Table
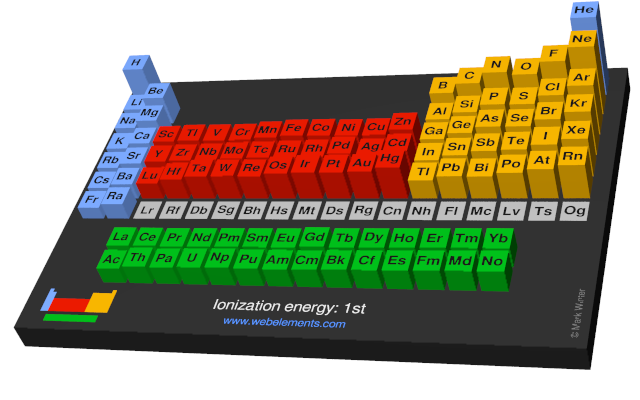
Webelements Periodic Table Periodicity Ionization Energy 1st
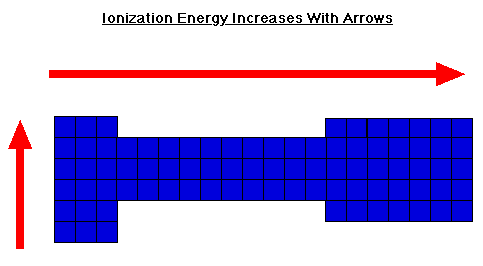
Ionization Energy
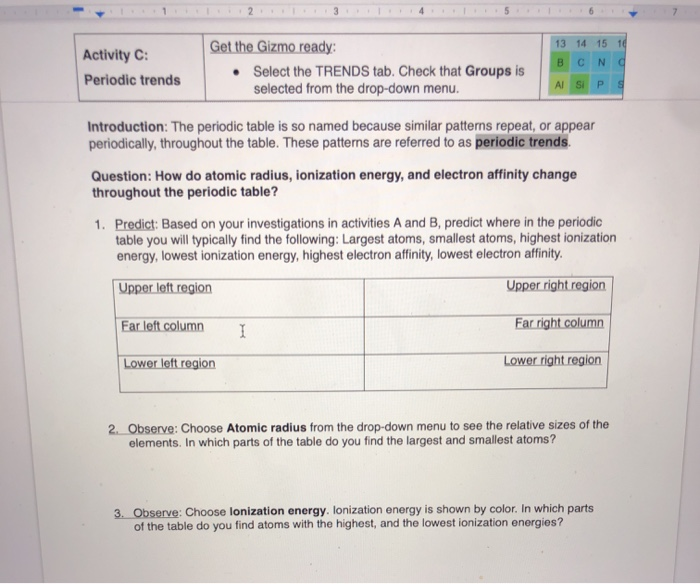
Solved 3 5 Get The Gizmo Ready 13 14 15 16 Activity C C

Periodic Table Ionization Energy Atomic Mass Electronegativity

How To Write Electron Configurations For Atoms Of Any Element

User Phaello Sandbox Chemistry Ionization Energy Wikieducator

Periodic Trends In Ionization Energy Ck 12 Foundation

Ionization Energy Trends Periodic Table Video Khan Academy

Chemical Bond Data

Atomic Periodic Properties
Energetics Of Ion Formation

Periodic Trends Chemistry Libretexts
Ionization Energy Chart
Ionization Energy

The Parts Of The Periodic Table

Periodic Table Trends Ionization Energy Youtube
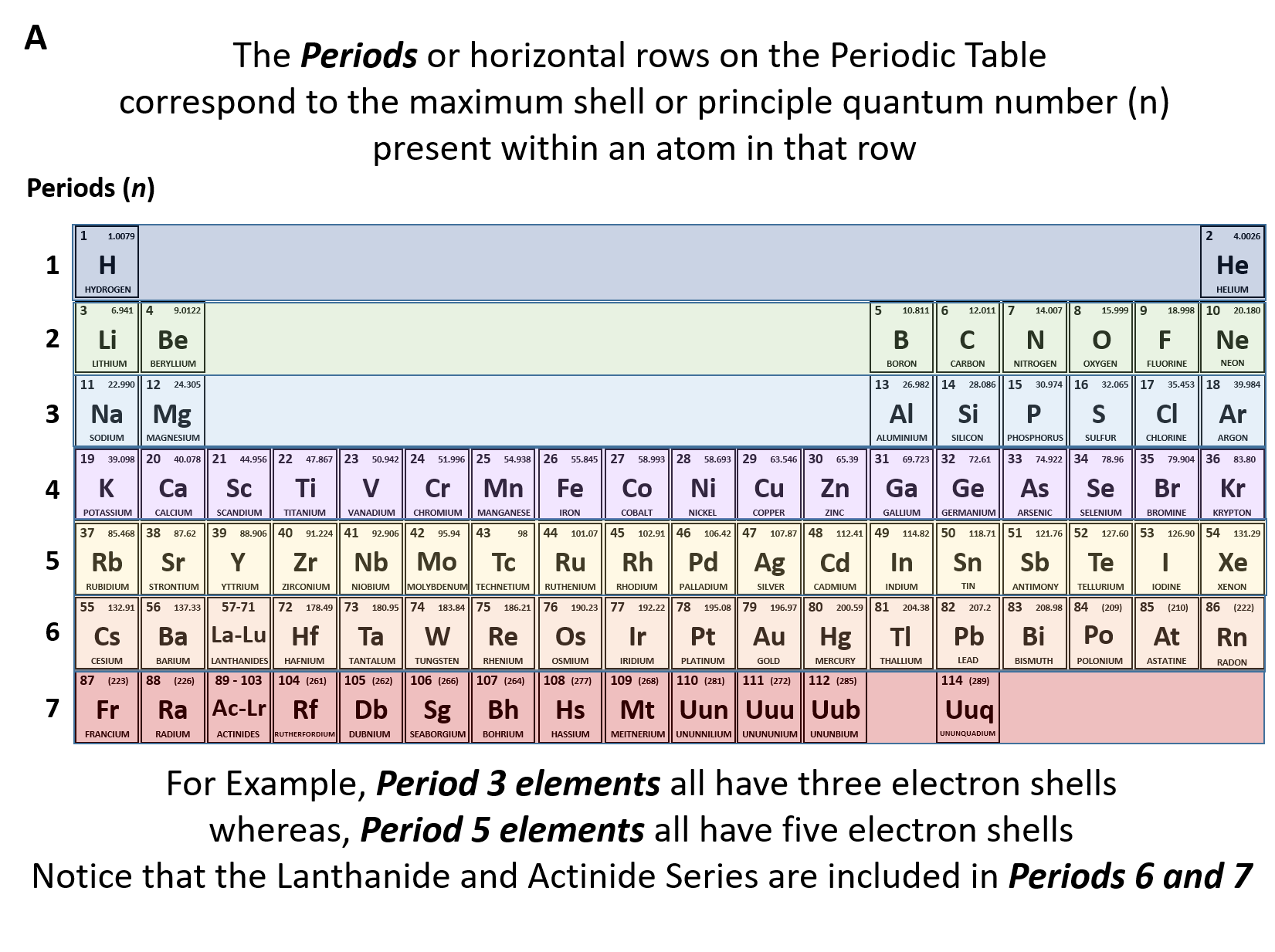
Ch150 Chapter 2 Atoms And Periodic Table Chemistry
how to find ionization energy
Source: https://goodttorials.blogspot.com/2014/08/how-to-find-ionization-energy-on.html
Posted by: schroederjace1953.blogspot.com

0 Response to "how to find ionization energy"
Post a Comment